Guiding the in-house path of an SDS: the approval workflow
The SDS (Safety Data Sheets), the documents containing the information required by the European REACH regulation on the hazardousness of chemical substances and mixtures, are delivered along the supply chain in support of the chemical products purchased.
In order to be able to carry out a proper chemical risk assessment and make complete and correct information available to end users, the SDSs received must be included in a process consisting of activities aimed at verifying,using, and sometimesenriching the data that are extracted from the SDSs. The approval process promotes and advances the SDSs in the workflow along with the collection of metadata that are managed at various stages of the process.
The planned activities require specific professional figures, sometimes from outside the company, to perform.
Guiding this process is therefore a priority, both because it is critical to define who and when is called upon to perform a given task and because activities must be fully defined and monitored.
With Share-SDS Drive the path taken by SDS in the company can be guided through the configuration of an approval workflow.
The workflow consists of a set of rules prepared for the purpose of coordinating the flow of activities that make up the entire process and is configured precisely to the specific business needs of each client.
Assuming that the needs of companies can differ widely, the basic ingredients of a workflow are:
Roles
The roles correspond to the professional figures that each workflow involves. They can be either internal to the company or external (e.g., Loader, Validator, Regulatory Advisor, Competent Physician, Laboratory Manager, HSE Manager, etc.).
Activities
The activities correspond to the tasks to be performed by each professional figure on each SDS (e.g., receiving and loading into the system, verifying regulatory compliance, enriching the metadata accompanying the SDS both chemical and business, disseminating to the company)
Actions
The actions correspond to the alternatives that each professional figure has, once the assigned tasks are completed. For example, an SDS can be accepted if it meets the requirements, be rejected if it presents criticalities, or be subject to specific treatment determined by the company.
Thanks to the workflow configuration, Share-SDS Drive allows different types of processes to be defined to tailor to the customer, defining one or more flows for the management of receiving SDSs for a new product (including R&D), receiving the update of an SDS already in the company, validation, approval from a central HSE body, receiving and approving from the 'H&S plant (where present), and finally widespread distribution to end users.
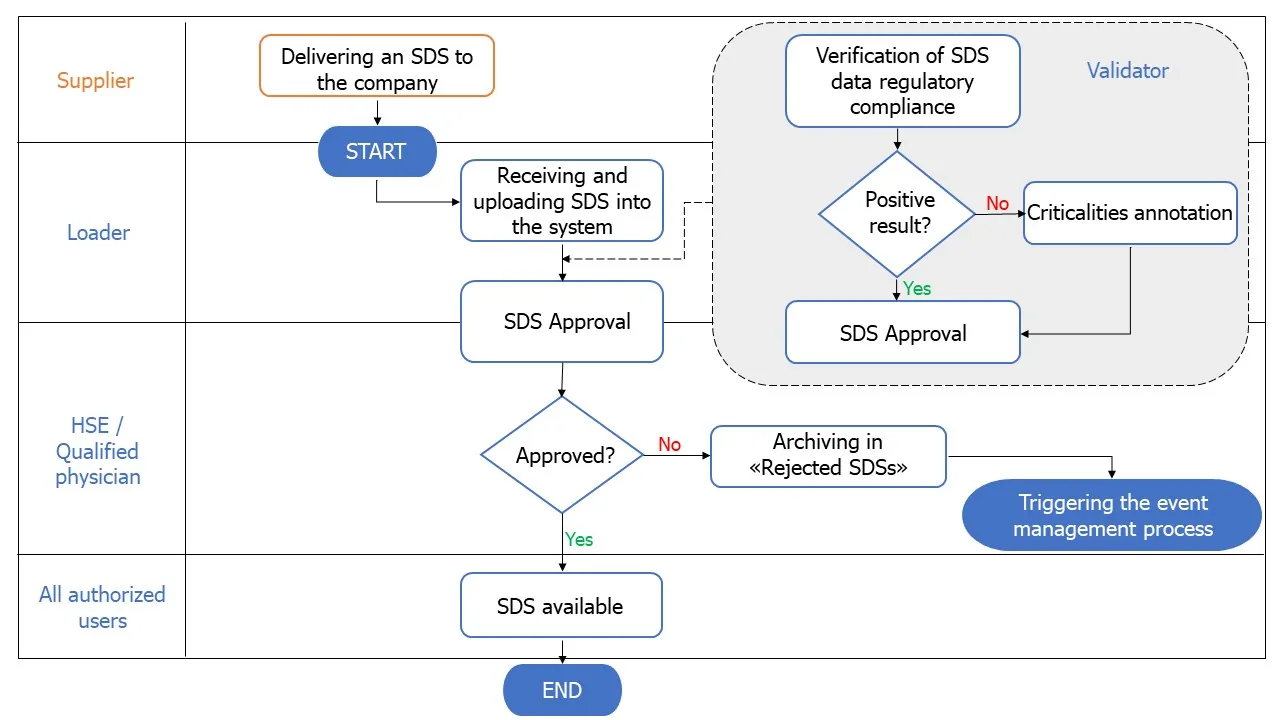
Example of a low-complexity workflow:
Once a workflow is configured, the company can coordinate the management of all SDSs and the enhancement of accompanying metadata from when they arrive at the company to when they are distributed to workers.
In addition to tracking, setting up a workflow via Share-SDS Drive allows you to know the processing status of each SDS by monitoring:
-
- Which professionals worked on the document and metadata,
- What activities were carried out (detailed on all metadata),
- At what time the activities were carried out ,
- What actions the activities have generated.
Functionality is also provided to verify the enhancement of all metadata throughout the entire pathway, allowing each activity performed on the document to be traced back.
The correct use of workflow features, combined with the ability to access the SDS according to different levels of authorization, is the basis for defining a selective view of the Safety Data Sheets.
In this scenario, each professional figure, depending on his or her role, will be able to be authorized to act on the document he or she is responsible for at the time it is needed. A user in the role of Validator will, for example, be able to see the SDS throughout the entire path; the Competent Doctor will be able to see the SDS only after it has been uploaded and validated; read-only enabled users, such as Department Heads and Workers, will see the SDS only at the end of the approval path provided by the company.
Learn how you can configure a Workflow to guide the path of an SDS in your company!
A path to learning through concrete examples and cases
Download the White Paper of the SDS Approval Workflow for more information, insights, and practical examples!
(Available only in Italian)
Our team is here to help you manage SDSs and keep track of hazardous substances!