
How to Address the Digital Transformation of the SDS Management Process.
How to Address the Digital Transformation of the Safety Data Sheet Management Process
Challenges, Opportunities and Essential Steps
Digitalization of data and processes has become essential for companies in every industry. When dealing with hazardous chemicals, Safety Data Sheet (SDS) management becomes a crucial element for worker safety, regulatory compliance and environmental protection.
Many companies still operate with cumbersome and disorganized processes for managing SDS, but the digital transformation of this process offers tremendous opportunities to improve operational efficiency and business security.
In this article, we will explore how to approach a project at digitalization of the SDS management process, examining the tangible benefits, common challenges and essential steps to follow. We will discover how digitalization not only simplifies the flow of information, but can also spur innovation and promote business sustainability. It is time to embrace a new perspective on digitalization processes, because what was once difficult to digitalize is now no longer difficult, and the achievable results can make a real difference.
The Benefits
1. Centralized and up-to-date management of SDSs and elimination of dispersion
Having a single repository where to receive, store, and manage the SDSs of the entire company makes it possible to keep track of these documents normally scattered among various company departments and allows them to be updated efficiently on an ongoing basis. It often happens that SDSs are held in various offices that keep copies without sharing them with other business functions. This leads to the generation of unnecessary duplicates, prevents the proper circulation of information and the efficient distribution of updates. Some companies that have undertaken the process of digitalization of SDSs have seen the number of documents decrease by as much as a third after the assessment project and subsequent cleaning.
2. Simple Sharing and Dissemination of SDSs
Centralizing the SDS repository also facilitates information sharing among different departments and business teams. All authorized users can access the latest versions of SDSs quickly and conveniently, ensuring a consistent and up-to-date flow of information.
3. Resource Optimization
Automation of processes reduces operational costs, enabling better allocation of human resources. This allows them to focus on higher-level activities such as data analysis, preventive measure planning, and staff training.
4. Increased Control of Hazardous Chemicals.
The company can more effectively monitor and assess potential hazards, making informed decisions for the safety of personnel and the environment and implementing appropriate preventive measures.
5. Increased Regulatory Compliance
A digital SDS management system facilitates monitoring and enforcement of current regulations. The ability to maintain traceability and history of actions taken enables companies to demonstrate compliance during inspections and audits. For example, the archive of obsolete SDSs or discontinued products to be maintained for at least 10 years can validate the presence of certain information in the company during a particular period, demonstrating actions taken over time.
6. Improvement of Commercial Compliance
Efficient control of hazardous chemicals in the company enables it to respond adequately to stakeholder requests regarding risk management and compliance with industry and/or private protocols.
7. Quick and Easy Search for Information in Case of Emergency
All operational personnel can immediately search for and retrieve the information needed to address a hazardous situation, enabling a timely and effective response. SDSs contain safety information and instructions for hazardous chemicals that should be consulted immediately in the event of an accident.
8. Stimulation of Innovation and Opportunities Related to Sustainability
More efficient management of hazardous chemicals makes it easier to adopt safer and sustainable alternatives, contributing to overall corporate responsibility. It also enables verifiability and measurability of progress.
Implementation strategies
The many benefits of digitalization of the SDS management process can be achieved by addressing and overcoming certain challenges that bring with them fruitful opportunities for growth and improvement.
Here are some tips on how to approach the project effectively.
1. Resource Management
Select a scalable and modular technical solution that fits the company's needs and resources
Break down the process of digitalization into steps: focusing on one step at a time helps avoid overload and better manage available resources
2. Technical Skills
Collaborate with vendors who offer technical support, training and consulting
To facilitate the overall success of the project, the solution provider must be able to work closely with the relevant IT staff
3. Budget Management
Demonstrating the value of digitalization to decision makers, clearly highlighting risks and benefits, can help obtain the necessary financial support
4. Inter-Departmental Accountability.
because the information contained in SDSs is useful to many business functions, it is critical to identify the department responsible for the project
in some contexts it is preferable for top management to sponsor the project
Essential steps
Let us now take a look at the key steps to address a path to digitalization of the SDS management process.
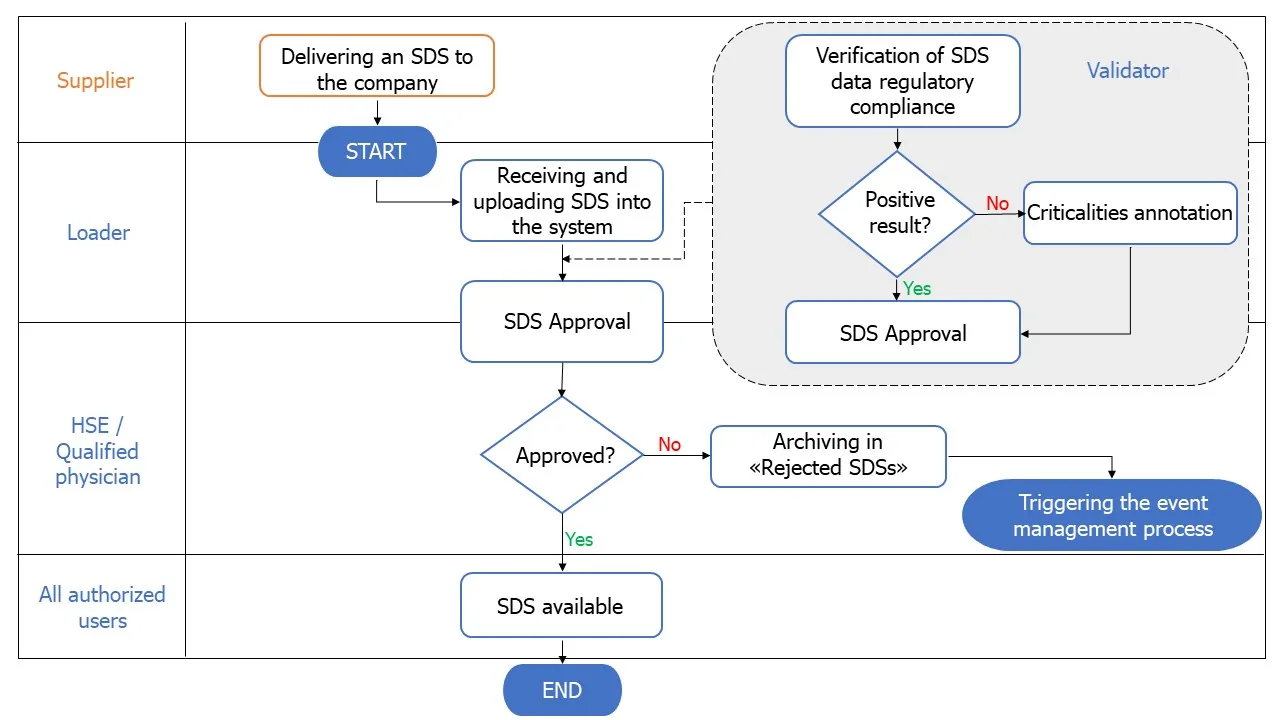
Organizing the Input of SDSs.
Establish a structured system to receive and record SDSs in an organized manner.
The process of receiving SDSs is crucial to the whole project at digitalization and is unfortunately often ungoverned and unstructured.
It is very important not to underestimate this first step that initiates the entire SDS journey in the company.
Indexing SDSs
Develop an extraction and indexing process to homogenize the data contained in SDSs and make them easily accessible and searchable, ensuring the correct classification of chemicals and associated hazards.
The extraction and digitalization of the data contained in SDSs should be approached with specialized automated tools as, if done manually, it has many disadvantages such as:
relevant work time
resources devoted to repetitive and unskilled work
difficulty in reading the contents of SDSs, which have different formats depending on the manufacturer
Lack of uniformity in data compilation by different operators
possibility of transcription errors
difficulty in continuously updating data
Archiving SDSs.
Collect SDSs in a single, up-to-date and controlled system to ensure that SDSs can be accessed and consulted by all relevant personnel.
Searching for documents and information contained in the centralized repository should be simple and clear for each figure involved, without over-information, but always in compliance with regulations. For example, for some business figures, the distribution of a simplified SDS can be considered.
Automating Specific Processes
Once the main processes are organized, one can move on to implement a workflow for the processes of validation, approval, and distribution of SDSs, with steps that can involve both internal and external roles within the company.
It is essential that theSDS repository be accessible according to an efficient and structured authorization system that allows for the necessary security of information, but at the same time is able to make SDS available to all workers, as mandated by the regulations.
Monitoring of MRSLs
The digitalization of data contained in SDSs can make it possible to automate a system of continuous comparison of hazardous chemicals in the company with reference MRSL (Manufacturing Restricted Substances List) lists.
To be able to do this, it is essential that the chosen solution includes management of public, private and/or industry MRSLs of interest to the company, cross-referencing them with data in the SDS archive.
Conclusions
The management of Safety Data Sheets For companies that deal with hazardous chemicals, it is a crucial element for safety, compliance, and business efficiency. The digital transformation of this process offers tangible benefits, including increased regulatory and commercial compliance, faster response to emergencies, and optimized use of resources.
However, this transformation is not without its challenges, including resource and budget management, technical expertise, and interdepartmental accountability. It is critical to demonstrate the value of digitalization to decision makers, highlighting the economic, environmental and social benefits, as well as the risks associated with poor SDS management.
What makes this transformation even more exciting is the potential offered by the new technologies available today, such as the spread of Internet connections and mobile devices, Cloud solutions and, more recently, Artificial Intelligence (AI) tools. These technologies generate a significant competitive advantage for those who adopt them. Indeed, they allow for simplification and significant savings in time and resources, as well as unprecedented access to vast amounts of information.
It is crucial to grasp a new perspective on digitalization processes such as SDS management. What seemed difficult to digitalizable and structure a few years ago can now become a driver of excellence. With the right approach and the right technologies, it is possible to transform SDS management and unlock its full potential.
Stefania Agiato
Every Software Solutions
Our team is here to help you manage SDSs and keep track of hazardous substances!









Recent Comments